Joint call Healthtech 2023
Based on the strength of the first Joint Call HealthTech, the Ministry of the Economy, the National Research Fund and Luxinnovation join forces again to further build the collaboration culture between companies, research and healthcare organisations. This includes matching HealthTech products or services at prototype stage with research and healthcare expertise to co-develop and validate these solutions at a clinical level for the benefit of patients and deliver leading innovation.
01 February 2023
31 March 2023
Contact
Desired goal of the call:
The development and validation of innovative digital health solutions benefiting the national healthcare (patients, healthcare professionals, payers), research and innovation ecosystems.
Objectives:
Incentivise collaborative R&D projects for improvement of health and care delivery focused on medical evidence, harnessing the opportunities of digital health (mHealth, telemonitoring, AI, personalised medicine...) in order to enable a patient centred healthcare.
The joint call intends to provide a financial incentive to consortia who have a specific digital product or service at prototype stage for which they wish to demonstrate its relevance and benefit for human health and society (the “project”).
Consortia may apply for funding at any of the three steps below, which can be considered as key R&D milestones towards market access for Health Technologies:
- Step 1: evidence of safety and performance (i.e. controlled environment), 1
- Step 2: evidence of effectiveness (i.e. real-life element),
- Step 3: evidence of favourable societal impact / evidence of value (e.g. calculated financial impact on health, social security, care, or impact of preventative medicine on education and work).
At the end of the project, the digital product or service shall be prepared to enter into the next stage or to market the solution after step 3. Commercialising the product immediately after the call is not a requirement, however, having a clear path to market is. This could include out-licensing of the digital product/service or fund raising for a further/final phase of clinical evidence in order to commercialise it.
Call topic:
Use of digital tools to improve prevention, diagnosis, monitoring or treatment of health conditions to improve public health or to increase patient autonomy, such as at home or in a care home environment
Products to be developed through this call must be innovative digital health solutions for human use that (1) are already at prototype stage and that can meet at least one of (2a) or (2b):
- (2a) are or will be regulated by the medical device regulation (MDR)2 or in-vitro medical device regulation (IVDR)3. (Examples: digital medical devices, digital health applications, connected medical devices)
- (2b) that will apply innovative data processing or analysis techniques on real world data from healthcare or care environments to prove a societal impact.
In line with national priorities seeking to develop personalised medicine further, and in line with strategic intentions to build a data innovation service platform, consortia will have to align to the following approaches:
Innovative clinical investigations
Centred on taking the current state of the art in clinical trials and refining their design and implementation through innovative means such as digital tools, smart wearables and sensors, electronic medical record analysis, and other precision medicine approaches. These clinical trials should bring added value to patients.
Multifactorial intervention strategies
Focused on designing and testing interventions that are not molecular in nature, for example through lifestyle and digital means. The goal is to improve health through innovative, participatory, and personalised intervention strategies, resulting in health-associated impact through interdisciplinary research.
General eligibility criteria and instruments of the joint call:
- Consortia are expected to include at least one eligible participating company and one FNR-eligible participating organisation. In the consortium, the contribution of the private and public parties should be as close to equal as possible, whereas no party shall bear more than 70% of the total project cost. Companies must fulfil the general eligibility criteria of article 2 of the RDI law4 and the respective criteria of the specific state aid scheme they apply for as set out in the R&D schemes. Research organisations must be eligible under article 3(2) of the FNR statute (Loi modifiée du 31 mai 1999 portant création d'un fonds national de la recherche dans le secteur public) and be registered at the FNR.
- The project must be in the field of experimental development, as defined in article 1 of the RDI law, and in line with the call topic.
- For experimental development activities under the joint call for projects, public institutions should comply with the general principles set forth in the FNR Guidelines, such as the formal requirements to qualify as PI (Principal Investigator) of an FNR-funded project and/or as supervisor of an FNR-funded PhD student, the FNR Research Integrity Guidelines, and the FNR data management plan, as well as those included in the FNR BRIDGES Programme description.
The FNR will fund the costs of the accredited research organisations in Luxembourg, up to 500.000€ per project covering all project specific costs. The Ministry of the Economy will co-finance costs borne by Luxembourg eligible companies up to ±700.000€ per project, using the R&D aid scheme.
Additionally, the Directorate of Health has the option to allocate 50.000€ grants to hospitals that are performing clinical research in projects selected as part of this joint call.
It is expected that the projects will be considered as experimental development projects. In this case, the maximum co-financing rates for companies through collaboration are as follows:
- Small company: 60%;
- Medium company: 50%;
- Large company: 40%.
Project durations are targeted for a 24 to 30 month period, with a 6 month extension to be requested if needed during the course of the project.
Upon justification regarding their liquidity needs, the Ministry of Economy may give a 35% upfront payment to companies leading a project selected in this Joint Call. For research and healthcare organisations FNR financial regulations apply.
Self-funded international or national partners are permitted to participate in the consortium.
Evaluation criteria and scoring system of the joint call:
The project proposals will be evaluated in a balanced manner based on the following criteria:
1. Relevance (33.3%)
- project idea; clarity and pertinence of the objectives
- level of innovation, including advance on state of the art
- soundness of the research approach and methodology, including the clinical or real-world evidence study
- likely favourable patient value of the technology
- due consideration of ethical and regulatory aspects
- ethical and legal governance model for a research data management and storage plan compliant with the required level of data security and privacy that is aligned with
- coherence and effectiveness of the work plan, including appropriateness of the allocation of tasks and resources
- realistic timing taking into account required authorisations
- realistic timing of patient recruitment, given the patient targeting and recruitment plans
- competences, experience and complementarity of the individual participants, as well as of the consortium and collaboration as a whole
- level of ambition in the collaboration and commitment of the participants in the proposed programme to pool national resources and coordinate their national competences, including a willingness to recruit on a multi-center national basis
- appropriateness of the management structures and procedures, quality of the risk management plan and soundness of the risk mitigation plan
- soundness of the data management plan built, when possible or pertinent, on existing data research infrastructures such as ELIXIR and others
- added value of the proposed product or service in terms of health and current clinical or care home care and/or care at home environment, as highlighted by the study
- strengthening the competitiveness and growth of involved companies by developing innovations addressing an identified market opportunity
- soundness of the business plan outlining a clear path towards an economic exploitation of the project results
- effectiveness of the proposed measures to exploit and disseminate the project results translated into assets, to communicate the project
- integration of health and disease data, sharing and reuse of data in the health field between researchers, companies and hospitals, while ensuring legal compliance on the use of the data
- laying the foundations of data-intensive digital health approaches for personalised medicine applications in Luxembourg and Europe
- strengthening the position of Luxembourg and the EU in the health technology domain and especially in the digital health domain through increased collaborations between public research, healthcare organisations and industry
Call process:
Submission process:
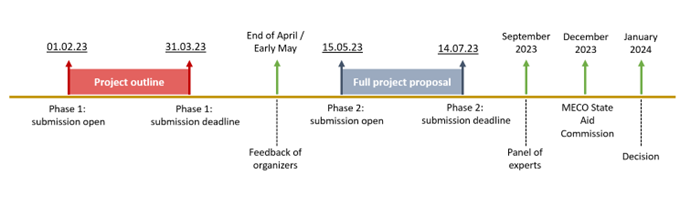
Phase 1: 1 Feb 2023 9h00 – 31 Mar 2023 14h00:Project outline (PO) to be submitted on the www.research-industry-collaboration.lu platform of Luxinnovation.
The PO shall provide information on:
- Project description;
- Project outcomes;
- Project starting point, available background demonstrating that the solution is at prototype stage;
- Plans to comply with GDPR requirements (DPO / DPIA), ethical (CNER) and clinical trials approvals (Ministry of Health);
- Intended research or development activities to gather medical evidence;
- Intellectual Property Rights for collaborative project proposals (in view of a draft collaboration agreement in phase 2);
- Preliminary project costs;
- Partners and CV of the main investigators (ORCID ID or LinkedIn Public Profile);
- For companies: Organisational chart of the group, 2021 and 2022 balance sheets and P&L accounts of the applicant and the group, cash-flow forecast.
Full project proposal (FPP) to be submitted by each project participant either to the Ministry of the Economy (Myguichet platform) for companies and to FNR (FNR Grant system) for accredited research organisations. The FPP as well as the financial appendices to be attachedd by each partner to the aid application can be downloaded from the platform www.research-industry-collaboration.lu.
The FPP shall provide information on:
- Detailed description of the research project;
- Different activities of the project (work packages);
- Description of the technical challenges and implementation of the project;
- Description of the expected outcome and the economic impact;
- Milestones;
- Timeline;
- Resources;
- Description of costs;
- Collaboration agreement (draft ready for signature) including agreement on intellectual property5;
- Medical certification and commercial path, highlighting the current stages of development of the technology and aim of the project (Step 1, 2 or 3);
- Draft clinical evaluation (clinical investigation, real-world evidence, protocol, usability study… in line with medical certification strategy and /or proof of value of the product/solution);
- Risk management and quality assurance;
- Proof that the sponsor of the clinical study has entered discussions with an insurance to cover damages resulting from the research and caused to persons participating in this trial (if applicable);
- GDPR aspects: data flow and ownership, delegations to data processors
Evaluation process:
Based on the Project Outlines (PO) and the appendices submitted via the research-industry-collaboration platform, the granting authorities in collaboration with Luxinnovation will check:
- Eligibility of all parties and co-funding capacity of the company;
- If project description is in line with the call topic;
- If the project objectives are in line with the objectives of the call.
Phase 2:
Full project proposals (FPP) prepared in Phase 2 will be reviewed by an independent expert panel (“panel”) that will assess FPPs from a scientific/technical and economic point of view. The panel will establish a ranking list based on the criteria set in the “Evaluation criteria and scoring system” section above. The highest ranked projects will be recommended for funding to the FNR and the Ministry of the Economy. All projects will undergo an additional consultation at the State Aid Commission. The decision on the company’s grant is subject to a further positive recommendation by the State Aid Commission.
A project can only be funded by a concurring decision of the FNR and the Ministry of the Economy.
The results of the evaluation will be communicated in January 2024, at the latest. Projects can start from February 2024.
Clinical investigations to develop and validate digital health solutions (i.e. medical devices), require an approval from the Ministry of Health and / or the CNER.6
The FNR and the Ministry of the Economy shall jointly examine the midterm and final deliverables of the projects of the consortiums selected by the Joint Call.
Joint Call HealthTech timeline:
References:
1 As per definition Art 2 (45) in Medical Device Regulation (EU 2017/245)
2 MDR reference text: https://eur-lex.europa.eu/eli/reg/2017/745
3 IVDR reference text: https://eur-lex.europa.eu/eli/reg/2017/746
4 Loi modifiée du 17 mai 2017 relative à la promotion de la recherche, du développement et de l’innovation
5 Any intellectual property (IP) rights that result from the collaboration should be allocated to the different collaboration partners in a manner which adequately reflects their contributions and respective interests in the project. The main IP terms of the collaboration agreement between the company and the public research institute should thereby comply with the “Framework for State aid for research and development and innovation (2014/C 198/01)”, paragraph 2.2.2. “Collaborations with undertakings”.
6 https://sante.public.lu/fr/espace-professionnel/recherche-biomedicale/procedure-autorisation-essai-etude-experimentation-clinique/index.html